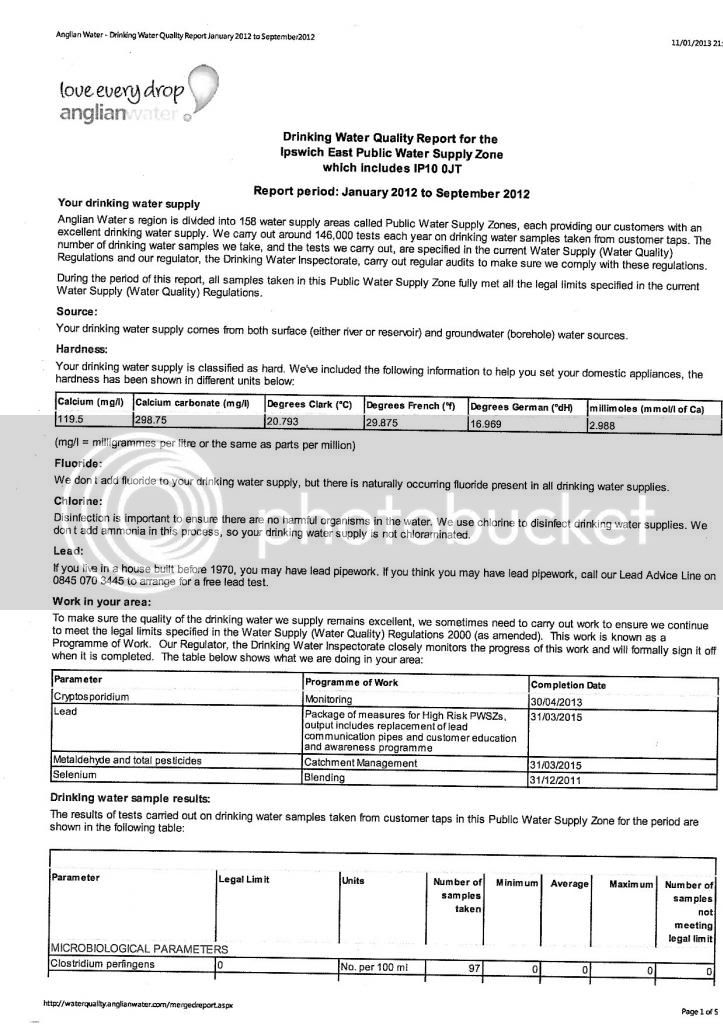
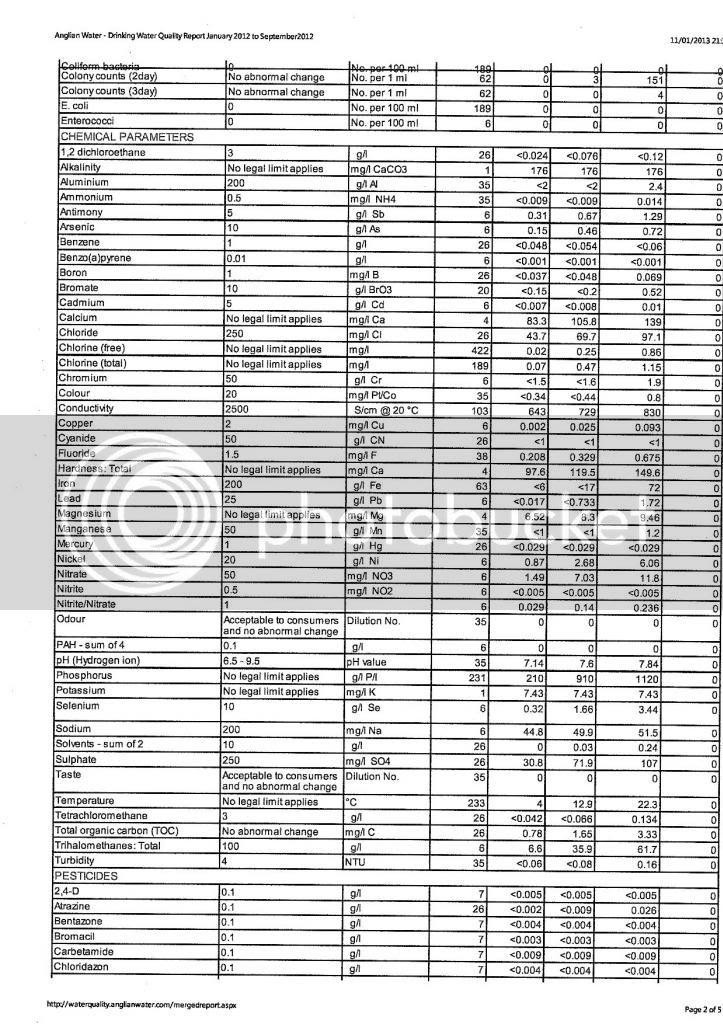
I assume you have the other water components like sodium, sulfate,and chloride. But I foresee a problem with regard to resolving the hardness value you are struggling with.
First, the alkalinity is reported "as CaCO3", so that is easy to resolve...enter that value in the "Alkalinity as CaCO3" box. The "Alkalinity as HCO3" is left blank.
That level of alkalinity is actually produced by a concentration of about 215 ppm HCO3 (bicarbonate) ions or around 106 ppm CO3 (carbonate) ions. In drinking water, the typical pH is typically below 8.5, so its more likely that the HCO3 ions are the dominant species in the water. CO3 only exists at high pH in water. These values were determined using the alkalinity conversion calculator in Bru'n Water software. Using the alkalinity value of 176 ppm as CaCO3 and an assumed pH of 7, the bicarbonate value is estimated. The CO3 concentration can be estimated by changing the pH to 14.
The hardness value is a tougher issue. Hardness is primarily the product of the calcium and magnesium concentrations in water. Since Temporary Hardness is always equal to Alkalinity, I can see that the reported hardness value is either a Total Hardness or Total Calcium Hardness. The problem is that some of the total hardness is probably from magnesium too and there is little information given to resolve the relative concentrations of Ca and Mg in the water with only a Total Hardness value. If that hardness value is Total Calcium Hardness, then it would be easy to convert that into an actual calcium concentration using the calculator in Bru'n Water. Ultimately, that 298 ppm (as CaCO3) value needs to be converted into the Ca and Mg concentrations that contribute to that hardness.
Hopefully the water report provides some additional information to help you resolve that issue. In the mean time, I suggest that you visit the Bru'n Water website and review the Water Knowledge page there to help you understand all this water stuff. Unfortunately the Bru'n Water version available for your review on that website does not include CRS, but the version sent to supporters does have that feature.
An important difference between Bru'n Water and the calculators shown on this site and others is that Bru'n Water actually guides your water treatment decisions to mesh with the composition of your mashing grist in order to produce an appropriate mash pH. Just adding acid or minerals without knowing their effect may not produce great results.