StevieDS
Regular.
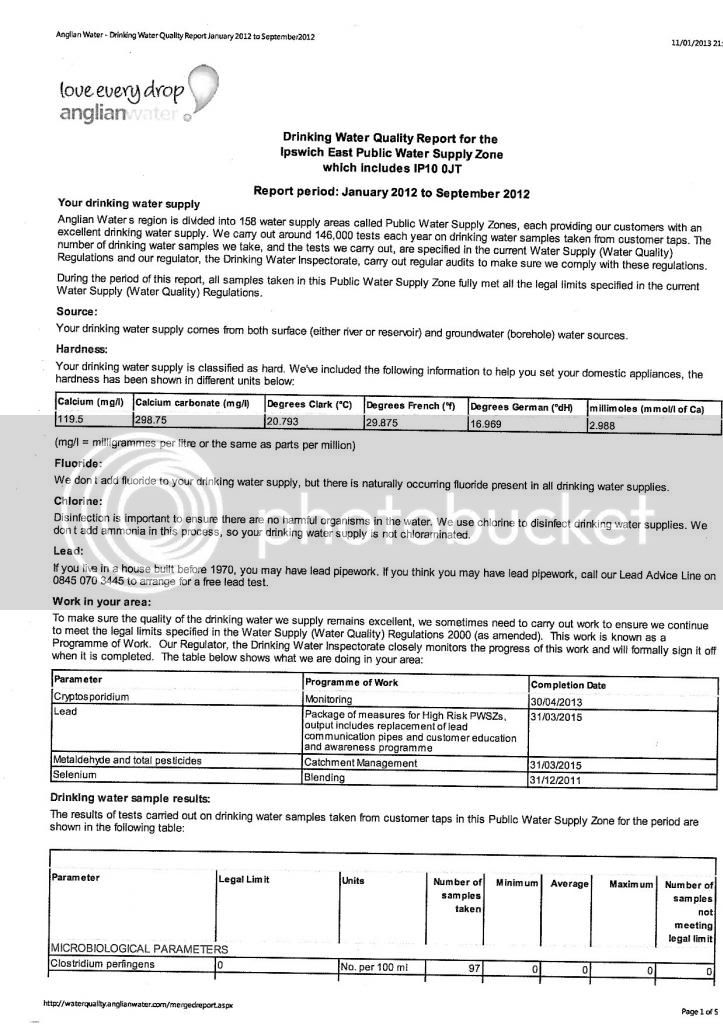
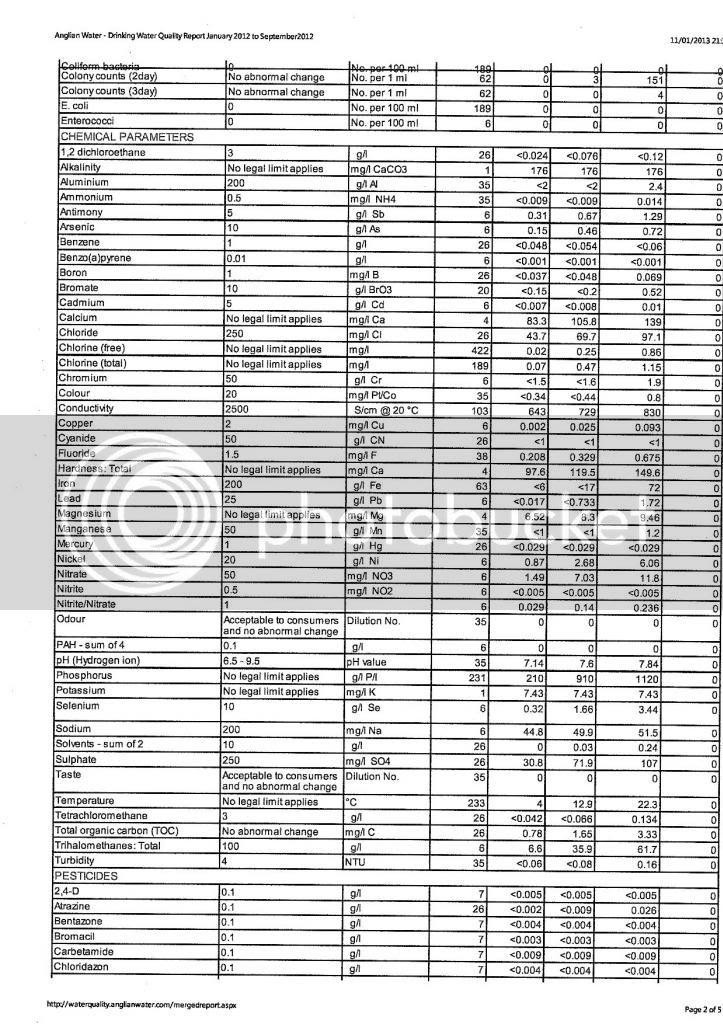
I've been messing around with a few water calculators online trying to get a decent water profile for my next brew. I received a water report from my local council but I'm not sure I'm reading it correctly.
I have the ppm values for the following minerals: sodium, potassium, sulphate, chloride and nitrate. That's fine, but for the more important ones such as calcium, magnesium, carbonate and bicarbonate its not quite as clear, to me anyway. The report doesn't give specific values for these, this is exactly what it says:
"This area has an average hardness of 34mg/l calcium (permanent) which is classed as moderately soft. This equates to 85mg/l CaCO3 (temporary)."
From this can I work out the ppm levels of Ca, Mg, CO and HCO?? :wha:
I have the ppm values for the following minerals: sodium, potassium, sulphate, chloride and nitrate. That's fine, but for the more important ones such as calcium, magnesium, carbonate and bicarbonate its not quite as clear, to me anyway. The report doesn't give specific values for these, this is exactly what it says:
"This area has an average hardness of 34mg/l calcium (permanent) which is classed as moderately soft. This equates to 85mg/l CaCO3 (temporary)."
From this can I work out the ppm levels of Ca, Mg, CO and HCO?? :wha: