You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Water hardness at 427ppm
- Thread starter Fore
- Start date

Help Support The Homebrew Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
This batch is reported to be slightly stronger then . . . replace 183 with 192 method remains the same . . .and I bet it doesn't remove as much as we are told it should :whistle: :whistle: :whistle:danb said:Cheers aleman Murphys AMS tech sheet says 192 reduced with 1ml/l.
Providing I stirred correctly its come out at 115mg reduction for 1ml so quite a bit under.
I'll do the test again stirring more and I'll leave it a few minutes before testing. Do the indicator drops affect the strength as I noticed the drops vary in size? Thanks
I'll do the test again stirring more and I'll leave it a few minutes before testing. Do the indicator drops affect the strength as I noticed the drops vary in size? Thanks
Fore
Landlord.
So thanks Aleman. I've clearly got a lot to learn when it comes to water treatment, but I did take some good basics from this.
I sit between 2 mountain ranges, the Voges & Black Forest, so I guess my water is affected by that. Last year the report read 435ppm, so it has improved a bit . There are lots of local Breweries here though, such as Kronenbourg (1664), so it can't be all bad.
. There are lots of local Breweries here though, such as Kronenbourg (1664), so it can't be all bad.
My basic understanding of PH and alcalinity was flawed, so some reschooling necessary. At least I have the pointers now. As I'm never going to be a chemist, I'll play it by ear. Seems that I might need to find a local source of RO, but I'll tackle that when I come to it. At least I now understand that this would be for dilution, and not substitution. Things are somewhat clearer : .
.
I sit between 2 mountain ranges, the Voges & Black Forest, so I guess my water is affected by that. Last year the report read 435ppm, so it has improved a bit
My basic understanding of PH and alcalinity was flawed, so some reschooling necessary. At least I have the pointers now. As I'm never going to be a chemist, I'll play it by ear. Seems that I might need to find a local source of RO, but I'll tackle that when I come to it. At least I now understand that this would be for dilution, and not substitution. Things are somewhat clearer :
 .
.AH HA! That explains it! I've been trying to work out where in the UK you were to have a hardness that high. Just a little request to everybody please fill in the Location part of your profile (User Control Panel -> Profile -> Location) even if it is just a general hint of where you are, it makes life easier to guess about local conditionsFore said:I sit between 2 mountain ranges, the Voges & Black Forest
Not necesarily a good thing either as a lot of the modern breweries use deionisation plants to strip everything out of the water to start with then add back what the need so that the water is a known constantThere are lots of local Breweries here though, such as Kronenbourg (1664), so it can't be all bad.
It's been a hard struggle for me over the years to get to grips with it even with a biochemistry degree . . .There is a lot of confusing stuff out there that appears sound but when you get to the nitty, gritty you find it's wrong, or only applicable in a limited set of conditions.My basic understanding of PH and alcalinity was flawed, so some reschooling necessary.
StevieDS
Regular.
Aleman said:It's been a hard struggle for me over the years to get to grips with it even with a biochemistry degree . . .There is a lot of confusing stuff out there that appears sound but when you get to the nitty, gritty you find it's wrong, or only applicable in a limited set of conditions.
This makes me feel a little less stupid
Fore
Landlord.
My OP opening paragraph did state my location, but I get the point, I'll update my profile.Aleman said:I've been trying to work out where in the UK you were to have a hardness that high.
I'm just digging into Alcalinity measurement now; already confused :wha:. I'll return with question if I can't clear these confusions myself. Thanks again.
YeahFore said:My OP opening paragraph did state my locationAleman said:I've been trying to work out where in the UK you were to have a hardness that high.
NickW
Landlord.
Sorry this is swaying off topic a tad.
I contacted asda the other day and they left a voice message on my phone stating that they can't and won't give me a report on their smart price water, and this was because it MAY be bottled at different sources. Bummer :hmm:
I contacted asda the other day and they left a voice message on my phone stating that they can't and won't give me a report on their smart price water, and this was because it MAY be bottled at different sources. Bummer :hmm:
They quote a typical analysis on the bottleNickW said:I contacted asda the other day and they left a voice message on my phone stating that they can't and won't give me a report on their smart price water, and this was because it MAY be bottled at different sources. Bummer :hmm:
While it MAY be bottled at different sources actual analysis over time has showed it to be remarkably stable, and close to the 'typical' profile.
User wallybrew has posted the analysis for smartprice on JBK several times IIRC
StevieDS
Regular.
May be different in England but over here the smart price water has no analysis on it. However another forum member posted a typical analysis after enquiring, see viewtopic.php?f=31&t=34935.
I can't vouch for the accuracy of this though.
I can't vouch for the accuracy of this though.
NickW
Landlord.
Cheers aleman. Nope, I've never seen an analysis on the bottle, not even a pHAleman said:They quote a typical analysis on the bottleNickW said:I contacted asda the other day and they left a voice message on my phone stating that they can't and won't give me a report on their smart price water, and this was because it MAY be bottled at different sources. Bummer :hmm:
While it MAY be bottled at different sources actual analysis over time has showed it to be remarkably stable, and close to the 'typical' profile.
User wallybrew has posted the analysis for smartprice on JBK several times IIRC
Fore
Landlord.
Would you believe it, today I received a letter from the water company to say that the water is to be sourced from a different water treatment centre, and the most significant change is that the hardness will more than half. What about that for timing; so chuffed  .
.
gazkilla
A hoppy middle aged man
After reading this I thought I'd get a Salifert kit. I got an alkalinity reading 2.96 x 50 =148 CACO3........but I haven't a clue what this means :grin: anybody??
rpt
Brewing without a hat
It means you can feed it into a water calculator along with the other values from your water report and work out what acids and minerals to add to your mash or boil for the style of beer you're brewing.
gazkilla
A hoppy middle aged man
Ok thanks,I found my water supplier was South Staffs water and it came from Burton upon Trent,I knew I moved here for a good reason :)
here's what I put in and it seems I just need some Carbonate Reducing Solution
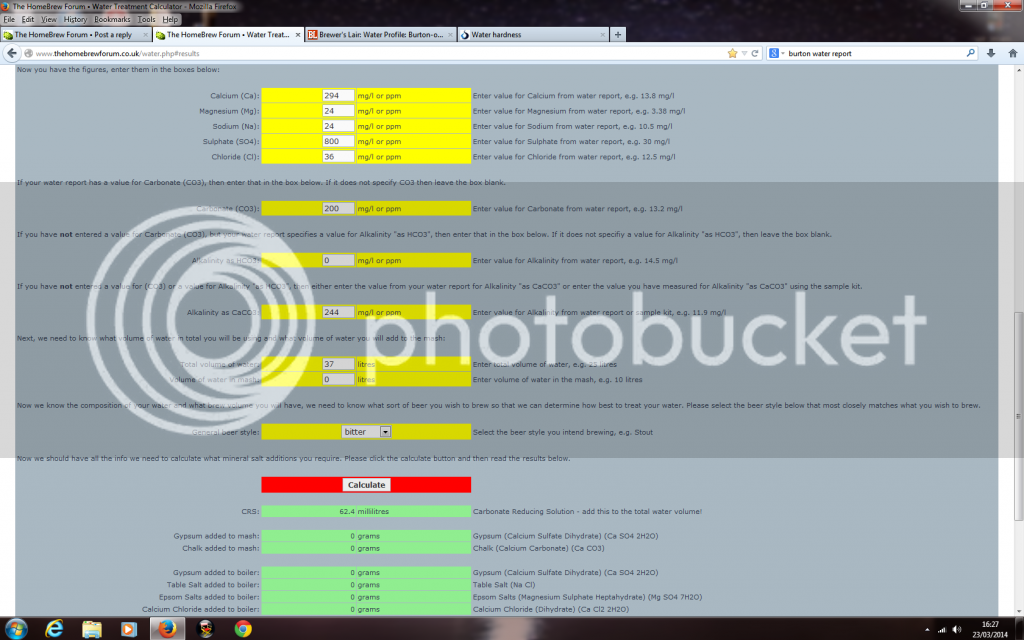
here's what I put in and it seems I just need some Carbonate Reducing Solution

That just does not add up, If you consider that the majority of alkalinity is in combination with calcium . . .then what is compensating for the sulphate . . .it isn't the residuals for the calcium together with teh sodium and potassium.gazkilla said:here's what I put in and it seems I just need some Carbonate Reducing Solution

Having run it through a different profiler, it does not appear to be way way way out of balance though
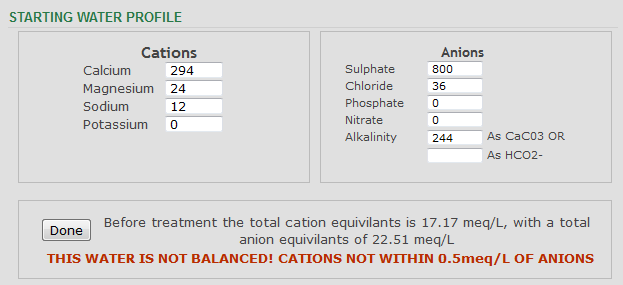
I'd bet that the sulphate level is much lower . . . and possibly the calcium level higher.
Too much sulphate for malty beers.
gazkilla
A hoppy middle aged man
I've emailed south staffs to give me a detailed report. I just googled Burton Upon Trent water report and rather stupidly just filled in the calculator from http://www.brewerslair.com/index.php?p=brewhouse&d=water&id=&v=&term=1 
 Only just realising that its a 'how to make your water up to Burton' guide.....I think :) Anyhow I'll await there reply. Thanks anyway,Aleman
Only just realising that its a 'how to make your water up to Burton' guide.....I think :) Anyhow I'll await there reply. Thanks anyway,Aleman 
gazkilla
A hoppy middle aged man
Got a phonecall off South Staffs water today and they gave me the following readings.
Hopefully these look better Aleman :)
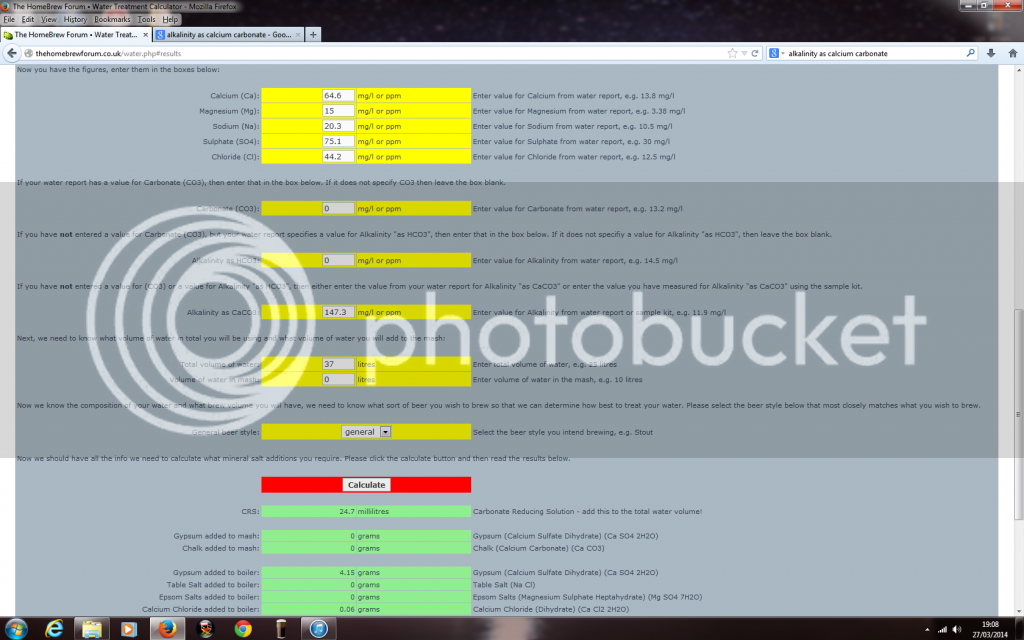
Hopefully these look better Aleman :)

Similar threads
- Replies
- 22
- Views
- 3K
- Replies
- 11
- Views
- 1K


