Lee Brown
Well-Known Member
- Joined
- Oct 2, 2019
- Messages
- 101
- Reaction score
- 20
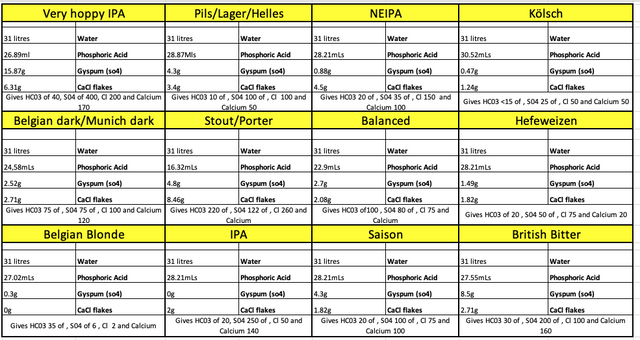
Thanks @Argentum. That's very useful.
So...
61/50 x 367 = 447.7 HC03
447.7-40 = 407.7 to remove (to hit 40 HC03)
407.7/61 = 6.68
6.68/12.1 x 31 = 17.11 mLs - to treat 31 litres of my water for an America style IPA.
Would that give me PH of 5.4 or 4.3?
I would then need to solve the 1:3 calcium to chloride ratio to get the right hoppy profile?
So...
61/50 x 367 = 447.7 HC03
447.7-40 = 407.7 to remove (to hit 40 HC03)
407.7/61 = 6.68
6.68/12.1 x 31 = 17.11 mLs - to treat 31 litres of my water for an America style IPA.
Would that give me PH of 5.4 or 4.3?
I would then need to solve the 1:3 calcium to chloride ratio to get the right hoppy profile?