strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,798
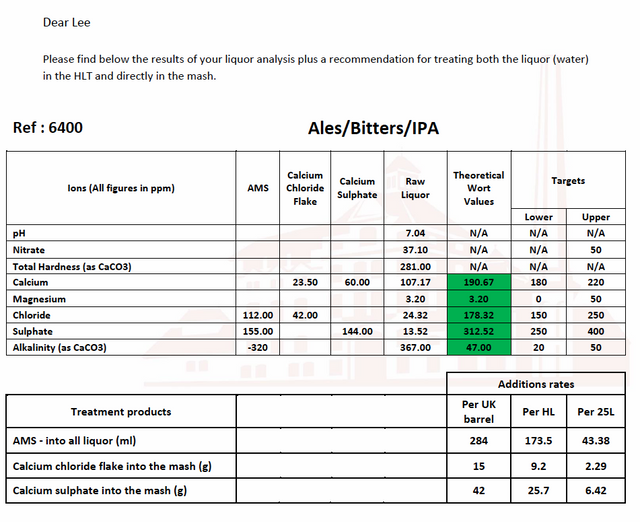
Well you weren't joking, that water's harder than Chinese calculus. I'll have a proper look at your info tomorrow mate but it looks to me like your buddy's recommendations are about right, and personally I'd be looking at dilution with soft water before treatment.