You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Beginners Guide to Water Treatment (plus links to more advanced water treatment in post #1)
- Thread starter strange-steve
- Start date

Help Support The Homebrew Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,798
That's not a first for Murphy's.your waters analyticals are impossible as stated
Argentum
Regular.
The constants in the above total hardness formula are derived from molecular weights as follow:
Molecular weights
----------------------
CaCO3 = 100.0869 g/mol
Ca = 40.078 g/mol
Mg = 24.305 g/mol
100.0869/40.078 = 2.49730 (for Ca++ ions)
100.0869/24.305 = 4.11796 (for Mg++ ions)
Molecular weights
----------------------
CaCO3 = 100.0869 g/mol
Ca = 40.078 g/mol
Mg = 24.305 g/mol
100.0869/40.078 = 2.49730 (for Ca++ ions)
100.0869/24.305 = 4.11796 (for Mg++ ions)
Argentum
Regular.
If it is Mash Made Easy spreadsheet, it is superb. But I still can see where I put a target alkalinity.
That's because there is simply no need to ever target alkalinity. What is instead to be targeted is ones desired mash pH. Generally most choose 5.4 pH at the target to shoot for here, but in my new way of seeing this 5.6 pH during the mash (as measured at room temperature) is an overall better target. Particularly if one will subsequently adjust post boil and cooling pH to 5.0- 5.2 pH (wherein this adjustment is to be done pre-boil, or alternately mid-way through the boil based upon pre-boil volume and specific gravity).
When people list ideal "target alkalinities", what they are really telling you is that if you begin with the listed "ideal target alkalinity" your mash pH will "hopefully" fall within the generally accepted range. For me the accepted range is 5.5 to 5.8 pH as measured at room temperature (or more technically precisely, 5.2 to 5.5 pH as measured at mash temperature). It is advised to measure mash pH at room temperature. That said, even with ATC 5.2 pH measured at mash temperature will be ~5.5 mash pH measured at room temperature. And both readings will amazingly be correct. Ditto for the case of 5.5 at mash temp and ~5,8 at room temp, wherein both of these are also correct readings. ATC is what assures that your reading will be factually correct regardless of temperature, even though to you they appear quite different. This is because Wort at high temperatures is factually more acidic than Wort at room temperature. ATC meters can't lie about the acidity and thereby pH that they are experiencing as temperature changes (although many of us explicitly want them to, and thereby believe that to be what ATC does). Instead, ATC equipped pH meters are seeing what factually is at any measurement temperature, and due to ATC they compensate for internal slopes such that what they report to us is what factually is (which is different for different temperatures). I know this confounds and baffles many minds, and many prefer the fantasy world wherein an ATC meter intentionally lies to our benefit and shows us that what it is seeing as 5.2 pH at mash temp is 5.5 pH (hiding that it is 5.2 pH), etc...
Last edited:
Lee Brown
Well-Known Member
- Joined
- Oct 2, 2019
- Messages
- 101
- Reaction score
- 20
Argentum
Regular.
So alkalinity not really important for colour? Just dial in your PH with salts and acid if needed then let the mash and yeast to the rest?
Almost. If you have a pH meter it is also desirable to adjust pH a second time, such that at post boil and cooling it reads 5.0 to 5.2 pH, Then let the yeast make beer.
Argentum
Regular.
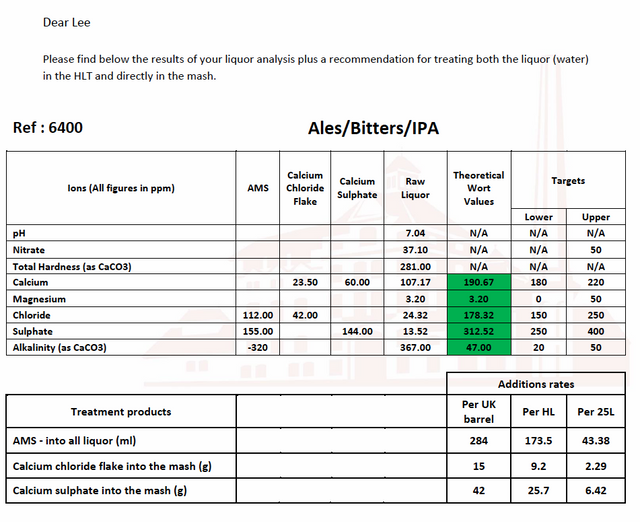
So this report from Murphy’s is wrong?
2.5 x 107.17 + 4.12 x 3.20 = ppm total hardness (as CaCO3) = 281 (so that part seems correct)
What is off is that first there is no sodium ion reported, and second either sodium is 62 ppm or alkalinity is not 367 ppm.
Lee Brown
Well-Known Member
- Joined
- Oct 2, 2019
- Messages
- 101
- Reaction score
- 20
2.5 x 107.17 + 4.12 x 3.20 = ppm total hardness (as CaCO3) = 281 (so that part seems correct)
What is off is that first there is no sodium ion reported, and second either sodium is 62 ppm or alkalinity is not 367 ppm.
It may be 62ppm. I’m guessing 48ppm as an average based on the website data from my water board.
I asked Murphy’s about sodium and they said they don’t report it for these. If I wanted to know my sodium it’d be another test and cost me more!
Argentum
Regular.
If your water authority draws from multiple sources and/or blends multiple sources at different rates at different times of the year that can be problematic with regard to reliable analyticals at any given time.
48 ppm Na does not toss your cation/anion mEq/L balance out all that terribly. If your sodium is between 48 and 62 ppm, then 367 ppm alkalinity is likely reliable.
48 ppm Na does not toss your cation/anion mEq/L balance out all that terribly. If your sodium is between 48 and 62 ppm, then 367 ppm alkalinity is likely reliable.
Argentum
Regular.
So alkalinity not really important for colour? Just dial in your PH with salts and acid if needed then let the mash and yeast to the rest?
In all cases, alkalinity is being controlled and altered to our benefit, and our only means to monitor the success or failure of this control and alteration is to measure the pH. Factually one never knows in advance that some particular ppm of alkalinity in the mash water will magically result in a properly mashed beer. The method of attempting to use alkalinity targets rather than pH targets is a band-aid kludge devised for those of us who don't have a clue as to pH. pH is driven by malt/grain acidity and the degree to which calcium and/or magnesium mineralization works upon the grist to lower it, in conjunction with mash water alkalinity and volume. High mash pH that is not follow-up adjusted downstream in the kettle leads to higher than intended colour. Both high and low mash pH bring with them a myriad of different flavours, flavour defects, etc...
Here is just one example of how using alkalinity targets may utterly fail us:
Person A sparges and splits mash and sparge water equally as to volume. Person B uses a no-sparge process and adds all water to the mash. Both measure their waters alkalinity at 120 ppm ahead of time, and both have read that for their identically chosen recipe the "ideal alkalinity target" just so happens to be 120 ppm. Both also adjust their water to identical target mineral ppm levels. Person B has just added twice as much alkalinity (as well as minerals) to his mash as has person A, and neither of them realize this fatal error because both have started with what some reference source has told them in advance is "ideal alkalinity" (and ideal mineral concentration) water. Neither has a clue as to the mash and sparge water volume ratios used by the person who generated their valued and treasured reference source. Both brew completely different beers while believing blindly that they are brewing the exact same beer.
Last edited:
Argentum
Regular.
Here is another way that alkalinity targets can fail us:
Joe Average goes to his local LHBS and buys 5 Kg. of Maris Otter. He makes a beer using alkalinity and mineral targeting found in a book he read and it turns out great. A year later he repeats the exact same process in "every" detail and it turns out to be disappointing. Joe is confused.
What Joe doesn't realize is that the DI_pH (deionized water mash pH) of his first lot of Maris Otter malt was 5.53 pH, and the same for his second lot was 5.82 pH.
10^-5.53/10^-5.82 = 1.95
What Joe missed was that his first base malt had almost twice as much inherent acid as did his second "identical" base malt. Water alkalinity and minerals work upon malt acidity. So by using the same alkalinity and mineral levels and water volumes and process his beers mashed at well differing pH's. And thereby they were different beers. One good, and one not so good.
Joe Average goes to his local LHBS and buys 5 Kg. of Maris Otter. He makes a beer using alkalinity and mineral targeting found in a book he read and it turns out great. A year later he repeats the exact same process in "every" detail and it turns out to be disappointing. Joe is confused.
What Joe doesn't realize is that the DI_pH (deionized water mash pH) of his first lot of Maris Otter malt was 5.53 pH, and the same for his second lot was 5.82 pH.
10^-5.53/10^-5.82 = 1.95
What Joe missed was that his first base malt had almost twice as much inherent acid as did his second "identical" base malt. Water alkalinity and minerals work upon malt acidity. So by using the same alkalinity and mineral levels and water volumes and process his beers mashed at well differing pH's. And thereby they were different beers. One good, and one not so good.
Lee Brown
Well-Known Member
- Joined
- Oct 2, 2019
- Messages
- 101
- Reaction score
- 20
Ohhhh, my head hurts now. I need a lie down.
So, in simple terms (for a dunder head like me), no matter what beer style I’m brewing, from Pilsner to Imperial stout, get your mash and water PH to 5.2-5.4 with acid reduction and the salts you want for the style (3:1 for hoppy ipa, 1:1 for balanced, 0:1 stout etc). Then let the malts and yeast take over. Maybe test ph into mash cycle to see if it is still in range, if not add a drop more acid?
I’m tying myself in knots here, but I’m trying to make my foray into this rabbit hole as painless as possible.
So, in simple terms (for a dunder head like me), no matter what beer style I’m brewing, from Pilsner to Imperial stout, get your mash and water PH to 5.2-5.4 with acid reduction and the salts you want for the style (3:1 for hoppy ipa, 1:1 for balanced, 0:1 stout etc). Then let the malts and yeast take over. Maybe test ph into mash cycle to see if it is still in range, if not add a drop more acid?
I’m tying myself in knots here, but I’m trying to make my foray into this rabbit hole as painless as possible.
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,798
While I agree that wort colour is not a very good basis for accurately predicting mash pH (and I acknowledge as much in the OP), in most cases it will be good enough to get you in the right area. I would suggest that estimating pH has similar problems though, it's purely theoretical due to the complexities of the chemistry involved and a vast number of variables. I'm not really sure that it's possible to accurately predict mash pH through a spreadsheet. I appreciate that it should be more accurate because it's taking more variables into account, but there's still too many to account for.In all cases, alkalinity is being controlled and altered to our benefit, and our only means to monitor the success or failure of this control and alteration is to measure the pH. Factually one never knows in advance that some particular ppm of alkalinity in the mash water will magically result in a properly mashed beer. The method of attempting to use alkalinity targets rather than pH targets is a band-aid kludge devised for those of us who don't have a clue as to pH. pH is driven by malt/grain acidity and the degree to which calcium and/or magnesium mineralization works upon the grist to lower it, in conjunction with mash water alkalinity and volume. High mash pH that is not follow-up adjusted downstream in the kettle leads to higher than intended colour. Both high and low mash pH bring with them a myriad of different flavours, flavour defects, etc...
This thread is after all a "beginners guide" and to be honest it's the method for water treatment that I current use. For a long time I used Bru'n Water, but I've since given up on that because targeting alkalinity using the simple method in the OP has worked very well for me, as confirmed by mash pH readings. There is a fairly wide margin for error, and brewing is probably a lot more forgiving than many of us imagine, and as long as the mash is somewhere between 5.2 and 5.8 I'm honestly not going to worry about it. Is there actually going to be a noticeable quality difference between a 5.8 pH mash and a 5.3 pH mash? I really doubt it.
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,798
I think this is getting way off topic here. This thread is for discussion of a simple method of water treatment and the last 4 or 5 pages have been full of fairly technical discussions which really are distracting from the OP and probably serve to confuse rather than enlighten anyone wanting to start this topic. I have no problems with the ongoing discussion, I just wonder if it might be better in a separate thread so as not to further confuse beginners?
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,798
In that case reread the OP and follow the instructions without getting yourself bogged down with details. Once you have a good grasp on the basics then you can look at the more advanced stuff.I’m tying myself in knots here, but I’m trying to make my foray into this rabbit hole as painless as possible.
Argentum
Regular.
One huge problem with all mash pH assistant software is that just as for Joe, the software has absolutely no clue regarding the actual DI_pH rating of the two "seemingly identical" base malts. And therefore it must guess as to the malts inherent acidity. The software's guess will be an averaged one reflected within its underlying programming, and will nigh on always be wrong with respect to your specific lot of base malt. The same is of course equally true for every other malt or grain in your recipe. All that one can hope for is that in a fuzzy logic sort of way a bunch of such errors work to cancel themselves out. Sometimes they do, and sometimes they do not.
Oops, I just read the above, and with due apology, I will refrain from further comment within this thread.
Oops, I just read the above, and with due apology, I will refrain from further comment within this thread.
Similar threads
- Replies
- 22
- Views
- 3K
- Replies
- 10
- Views
- 1K
- Replies
- 2
- Views
- 1K